One year after the nation was brought to a near-standstill by the coronavirus, President Joe Biden pledged in his first prime-time address Thursday night to make all adults eligible for vaccines by May 1 and raised the prospect of “independence from this virus” by the Fourth of July. He offered Americans fresh hope and appealed anew for their help.
AP: More Minnesotans eligible for coronavirus vaccine
Gov. Tim Walz announced Tuesday that Minnesota is expanding eligibility for the coronavirus vaccine after reaching its goal of inoculating at least 70% of people 65 and older.
AP: Fully vaccinated people can gather without masks, CDC says
Fully vaccinated Americans can gather with other vaccinated people indoors without wearing a mask or social distancing, according to long-awaited guidance from federal health officials.
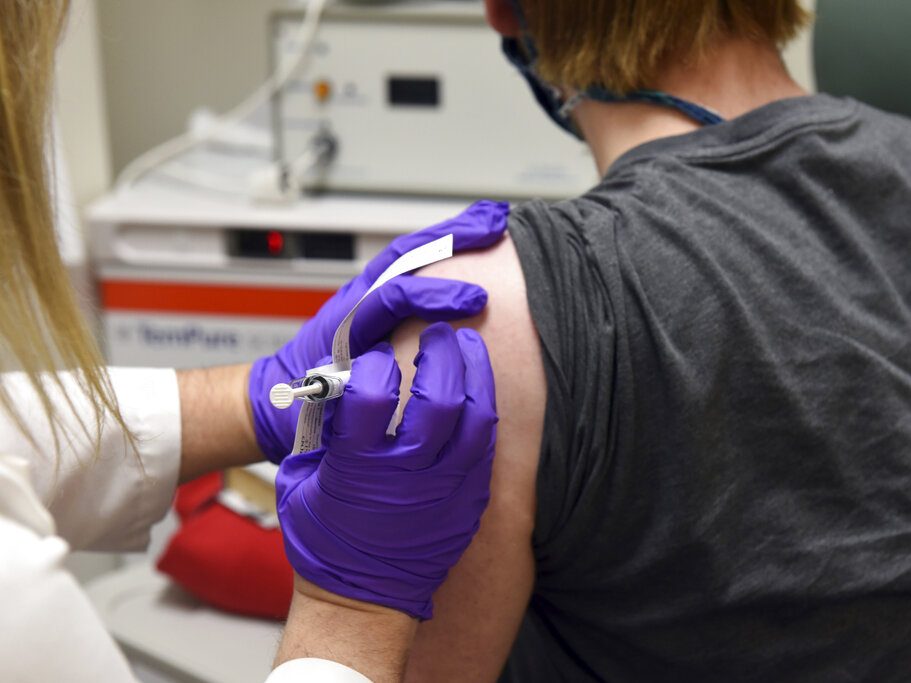
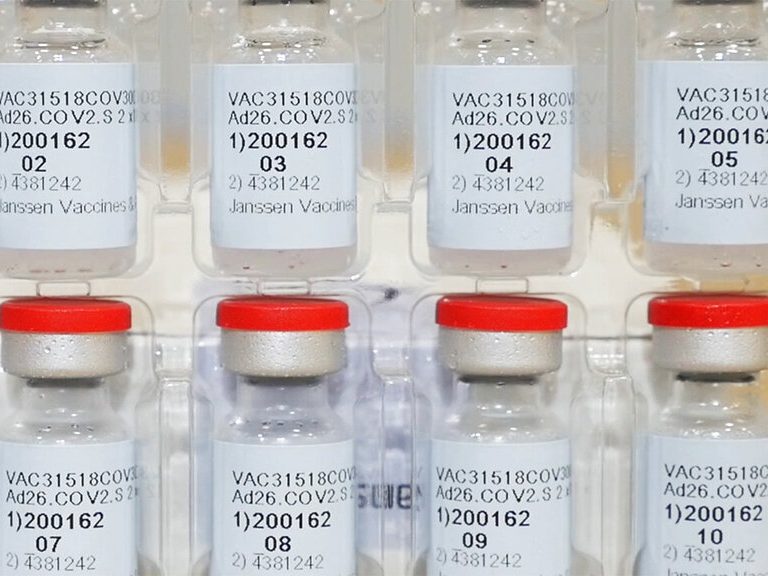
AP: J&J’s 1-dose shot cleared, giving US 3rd COVID-19 vaccine
The U.S. is getting a third vaccine to prevent COVID-19, as the Food and Drug Administration on Saturday cleared a Johnson & Johnson shot that works with just one dose instead of two.
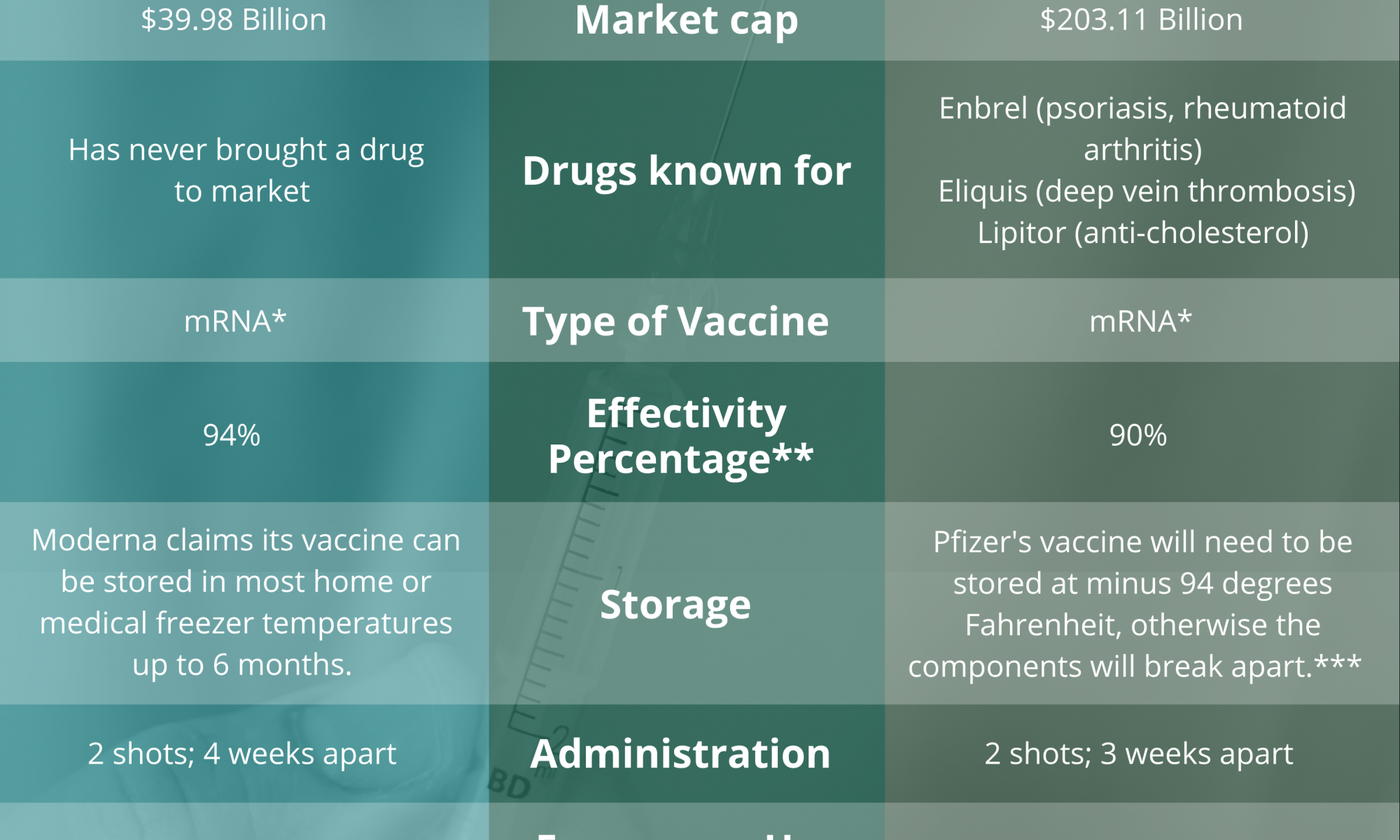
INFOGRAPHIC: Moderna vs. Pfizer COVID-19 vaccines
With the recent approval of two COVID-19 vaccines for emergency use authorization in the United States, Designer Lauren Dettmer shows what you need to know about the two leading vaccines from biotechnology companies Moderna and Pfizer.
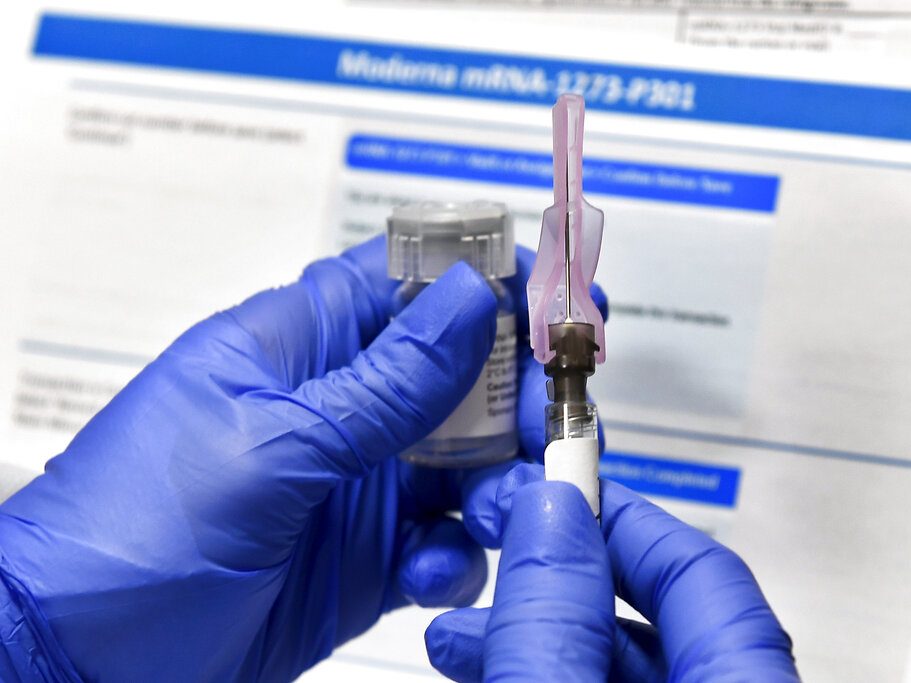
AP: US clears Moderna vaccine for COVID-19, 2nd shot in arsenal
The U.S. added a second COVID-19 vaccine to its arsenal Friday, boosting efforts to beat back an outbreak so dire that the nation is regularly recording more than 3,000 deaths a day.
AP: Walz eases restrictions on gyms and businesses, allows elementary schools to reopen
Minnesota Gov. Tim Walz announced plans Wednesday to get children back into elementary schools and ease some restrictions on fitness centers and other businesses that were shuttered last month to curb the spread of COVID-19, though bars and restaurants will remain closed for indoor service through the holidays.
AP: US allows emergency COVID-19 vaccine in bid to end pandemic
The U.S. gave the final go-ahead Friday to the nation’s first COVID-19 vaccine, marking what could be the beginning of the end of an outbreak that has killed nearly 300,000 Americans.
AP: UK authorizes Pfizer coronavirus vaccine for emergency use
Britain authorized a COVID-19 vaccine for use Wednesday, greenlighting the first shot backed up by rigorous scientific review. The first vaccinations are expected within days — a major step toward eventually ending the pandemic.
AP: Heading home for the holiday? Get a virus test, colleges say
As college students prepare to go home for the holidays, some schools are quickly ramping up COVID-19 testing to try to keep infections from spreading further as the coronavirus surges across the U.S.
AP: Minnesota shuts more businesses as COVID-19 spread soars
Minnesota Gov. Tim Walz on Wednesday imposed four weeks worth of new COVID-19 restrictions as the spread spiked to an all-time high, shutting down bars, restaurants and fitness centers, while pausing social gatherings and organized amateur sports.
AP: Minnesota to close bars, restaurants, gyms for 4 weeks
Minnesota Gov. Tim Walz plans to announce new COVID-19 restrictions on Wednesday that will shut down indoor dining at bars and restaurants, close gyms and fitness centers, and put organized indoor youth sports on hold for four weeks.
INFOGRAPHIC: Swab vs. saliva COVID-19 tests
St. Thomas announced it will begin offering free on-campus saliva COVID-19 tests for asymptomatic people on Wednesday, Nov. 18 and Thursday, Nov. 19. Design Manager Maggie Stout explains the difference between swab and saliva testing.
St. Olaf becomes second MIAC school to nix winter sports
St. Olaf College joins neighboring Carleton College as the second MIAC school to cancel the 2020-21 winter sports season for varsity athletic teams due to the ongoing COVID-19 pandemic. Sports Editor Joey Swanson and Production Editor Justin Amaker have the story.
AP: Pfizer says COVID-19 vaccine is looking 90% effective
Pfizer said Monday that an early peek at the data on its coronavirus vaccine suggests the shots may be a robust 90% effective at preventing COVID-19, putting the company on track to apply later this month for emergency-use approval from the Food and Drug Administration.